Abstract
INTRODUCTION: Therapeutic advances have led to improved survival outcomes in patients (pts) with AML. Consolidation therapy helps to eradicate residual leukemia and prevent relapse. However, relapse remains a major concern and contributes to suboptimal outcomes in pts with newly diagnosed AML who attain remission following induction chemotherapy (IC). Strategies for maintenance therapy (MT) attempt to prolong AML remission and extend survival. MT approaches have included chemotherapy, hypomethylating agents (HMAs), and targeted small-molecule drugs. Overall, the evidence in favor of MT is limited. The oral formulation of azacitidine (Oral-AZA), an HMA, is the first and only MT to demonstrate significant overall survival (OS) and relapse-free survival (RFS) benefits in pts with a broad range of AML subtypes (Wei, NEJM 2020), leading to regulatory approvals in the United States (2020), Canada (2021), and European Union (2021). This study aimed to determine in real-world practice whether the use of MT after IC, with or without consolidation, conferred any clinical advantage before Oral-AZA became available.
METHODS: This study included pts in the US-based Flatiron TM Health cancer-specific electronic health record-derived database diagnosed with AML between January 2014 and December 2020. Eligible pts in this study obtained remission (< 5% bone marrow blasts) from first-line (1L) induction chemotherapy or venetoclax (VEN)-based therapy, had not previously taken Oral-AZA or any clinical study drug, and did not undergo transplant prior to relapse. Pts were grouped into 2 cohorts: Cohort A comprised pts who did not receive MT, and Cohort B comprised pts who did receive MT. RFS and OS were estimated using Kaplan-Meier (KM) methods. Multivariate Cox regression models that retained baseline (BL) characteristics (age, sex, BMI, ECOG performance status [PS], and cytogenetic risk) were used to examine the relationships between use of MT and relapse (> 5% bone marrow blasts), and MT and survival.
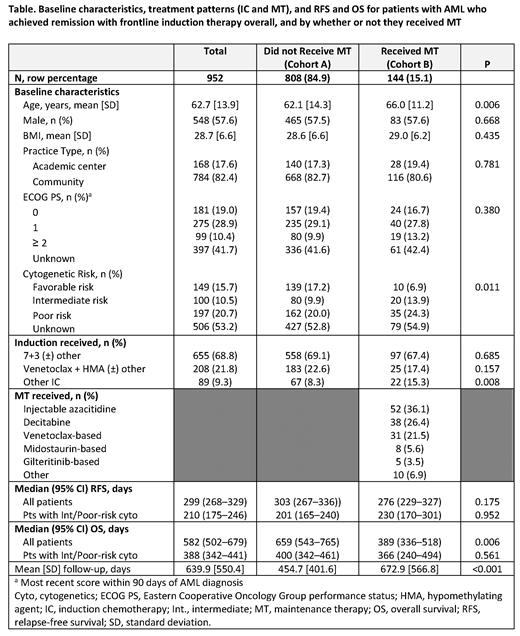
RESULTS: A total of 952 pts met the selection criteria: 808 pts (84.9%) in Cohort A and 144 pts (15.1%) in Cohort B. The most commonly received MTs for pts in Cohort B were injectable AZA (34.7%; n = 50), decitabine (24.3%; n = 35), and VEN-based regimens (21.5%; n = 31). Cohorts A and B were comparable for sex, BL BMI, ECOG PS score, and practice type (Table). Pts who did not receive MT (Cohort A) were younger (mean age 62.1 vs 66.0 years; P = 0.006) and more commonly presented with favorable cytogenetic risk (17.2% vs 6.9%; P = 0.011) compared with pts who received MT (Cohort B).
The percentage of pts receiving IC in a 7+3 regimen was similar between Cohort A and Cohort B (69.1% vs 67.4%, respectively; P = 0.685), as were the proportions of pts receiving VEN + an HMA (23.3% vs 17.4% P = 0.118).
The KM analysis indicated that although median RFS of Cohort A was longer than Cohort B, differences between cohorts were not statistically significant (303 vs 276 days, P = 0.175). In a subanalysis of pts with intermediate/poor cytogenetic risk, median RFS was 201 days for Cohort A and 230 days for Cohort B (P = 0.952). In the multivariate Cox model, treatment with MT was not a significant predictor of improved RFS vs no MT (hazard ratio [95% CI]: 0.97 [0.79, 1.20]). Age and cytogenetic risk were significantly associated with RFS.
The median OS was 659 days for Cohort A and 389 days for Cohort B (P = 0.006). In the subanalysis excluding pts with favorable or unknown cytogenetic risk, median OS was 400 days for Cohort A and 366 days for Cohort B (P = 0.561). A multivariate Cox regression model suggested that treatment with MT was not a significant predictor of improved OS (hazard ratio [95% CI]: 0.88 [0.70, 1.10]). Age, sex, ECOG PS, and cytogenetic risk were all significantly associated with OS.
CONCLUSIONS: These Flatiron data provide real-world evidence that despite decades of study, optimal MT in AML had remained elusive. Prior to approval of Oral-AZA, the most common MT options for pts with AML in first remission after 1L induction therapy were limited and appeared to lack clinical benefit. Pts with AML need an MT that prolongs remission and improves long-term survival. Further research is needed to elucidate the benefits and disadvantages of different MT options and may lead to establishment of optimal MT as standard of care.
Potluri: Bristol Myers Squibb: Consultancy. Rotter: SmartAnalyst Inc.: Current Employment. Chen: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal